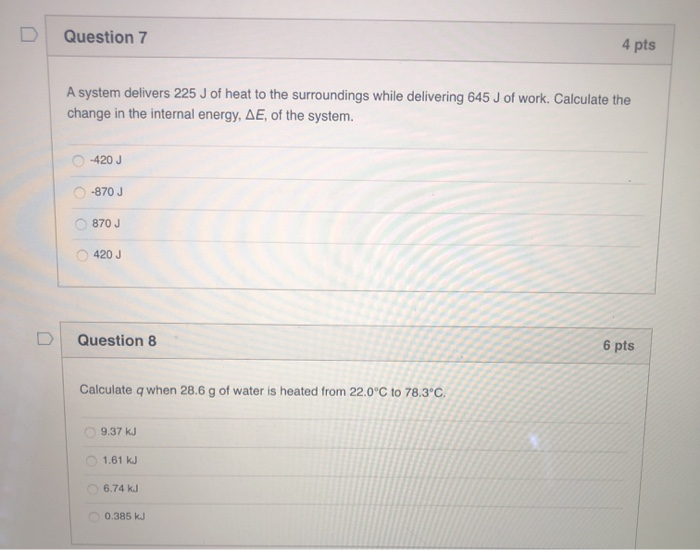
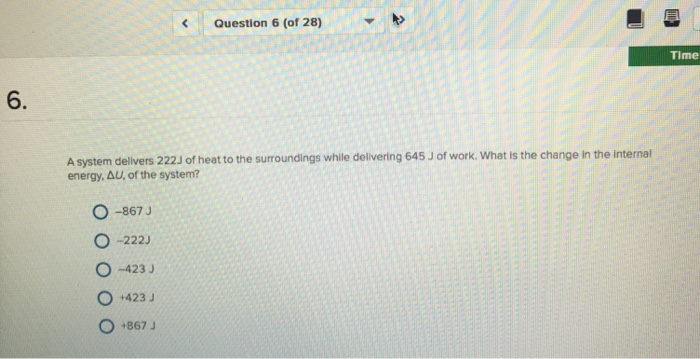
A System Delivers 225 J Of Heat
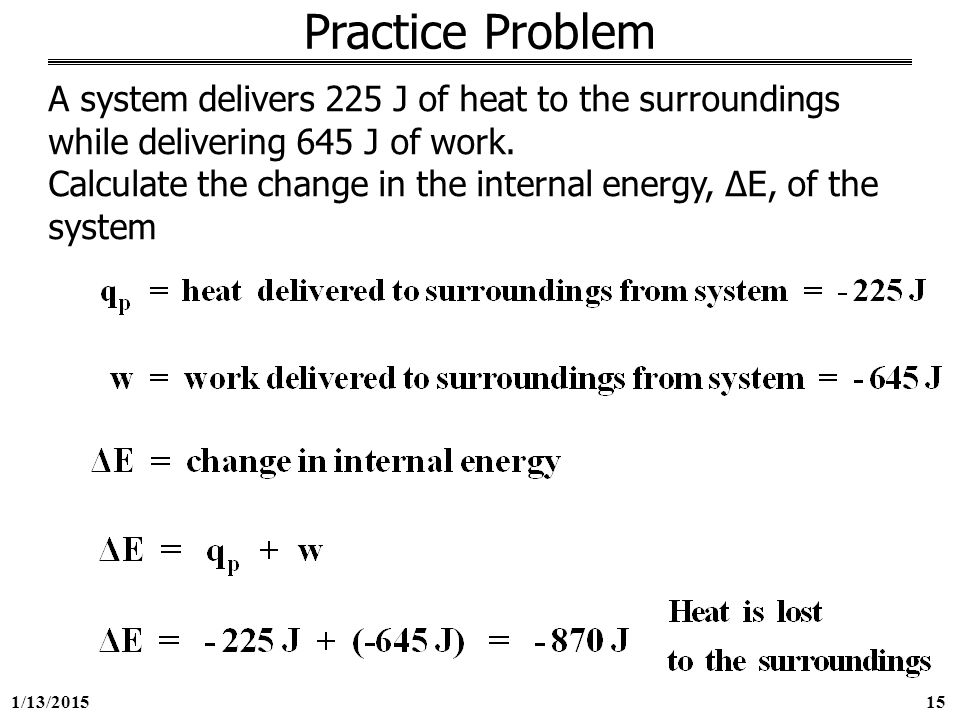
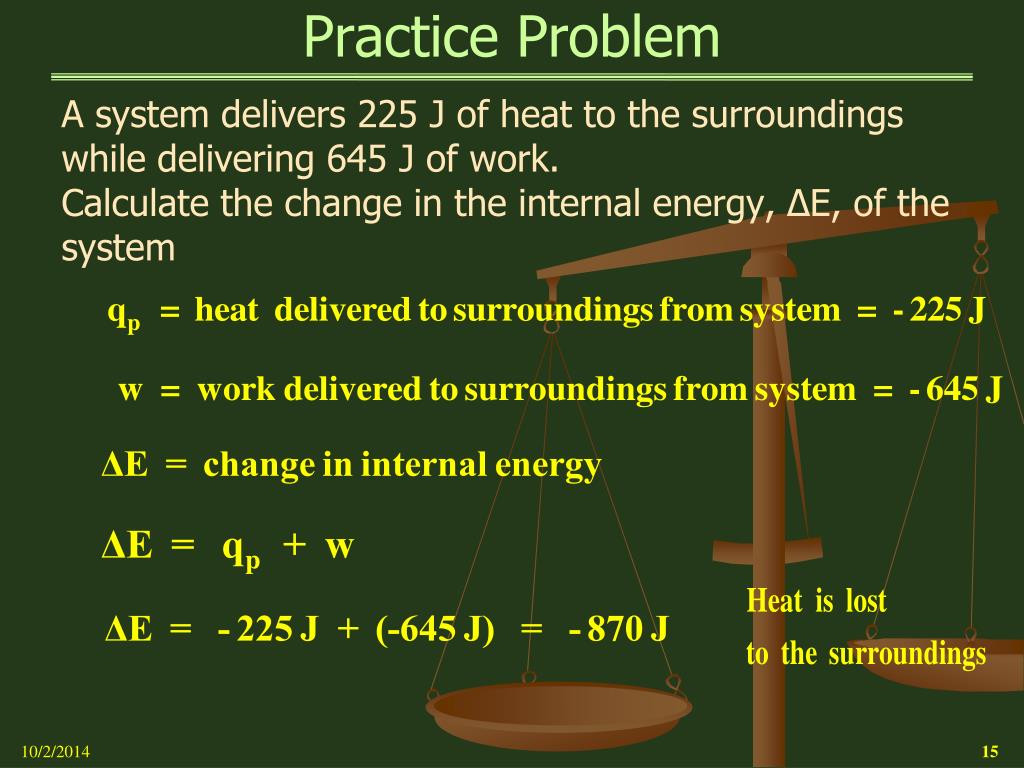
A system delivers 225 j of heat. Calculate the change in the internal energy ΔE of the system. A-420 J B 420J C -870 J D 870 J E -225 J 35. A system delivers 225 J of heat to the surroundings while delivering 645 J of work.
Calculate the change in the internal energy ΔE of the system. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. Asked Sep 2 2019 in Chemistry by Carmenon.
A system receives 575 J of heat and delivers 424 J of work. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. A system delivers 225 J of heat to the surroundings while delivering 645 J of work.
In which one of the following reactions would you expect ÎH to be substantially greater than ÎU ie. Calculate the change in the internal energy AE of the system. A system delivers 225 J of heat to the surroundings while delivering 645 J of work.
A system delivers 225 J of heat to the surroundings while delivering 645 J of work. In which one of the following reactions would you expect ΔH to be substantially greater than ΔU. A system delivers 225 J of heat to the surroundings while delivering 645 J of from CHY 121 at University of Maine.
Chemistry questions and answers. Claculate the change in internal energy δe of the system. A system delivers 225 J of heat to the surroundings while delivering 645 J of work.
A system absorbs 225 j of heat from the surroundings and delivers 645 j of work. Calculate the change in the internal energy E of the system.
A system delivers 225 J of heat to the surroundings while delivering 645 J of work.
Calculate the change in the internal energy ΔE of the system. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. A system absorbs 225 j of heat from the surroundings and delivers 645 j of work. Calculate the change in the internal energy ΔE of the system. Calculate the change in the internal energy DE of the system. Calculate the change in the internal energy AE of the system. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. Chemistry questions and answers.
A system delivers 225 J of heat to the surroundings while delivering 645 J of work. In which one of the following reactions would you expect ΔH to be substantially greater than ΔU. Calculate the change in the internal energy ΔE of the system. Chemistry questions and answers. Calculate the change in the internal energy ΔU of the system. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. A system delivers 225 J of heat to the surroundings while delivering 645 J of work.





























Post a Comment for "A System Delivers 225 J Of Heat"